 3449
3449
 2018-02-05
2018-02-05
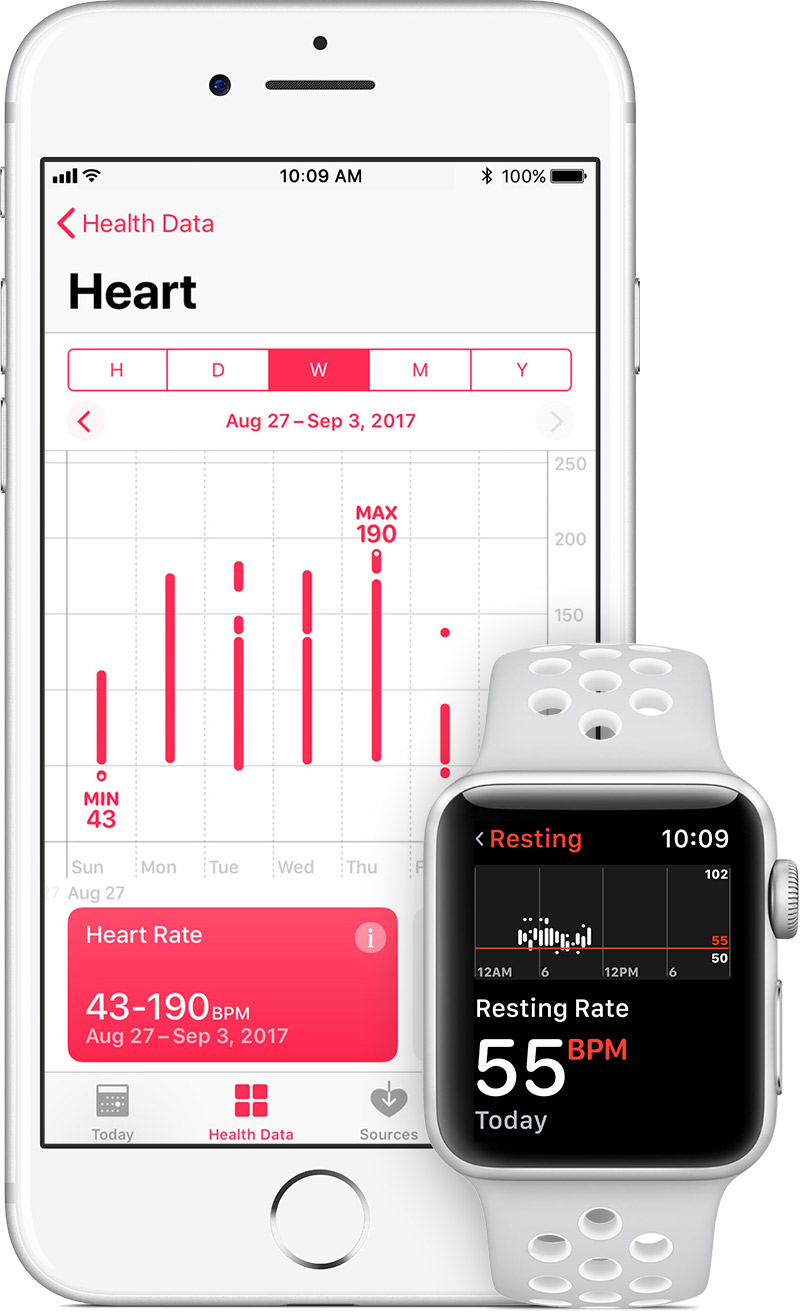
Back in November, Apple asked Apple Watch users if they wanted to take part in a study called the Apple Heart Survey being conducted by Apple and Stanford Health. Now, Apple is telling those who opted-in that the study has begun. Tapping on the notification takes participants to the Heart Study app where they must confirm that they are "comfortable" speaking and writing in English, and haven't been diagnosed with Atrial fibrillation or Atrial flutter. In addition, participants cannot be on blood thinners like Coumadin, Pradaxa, Xarelto, Eliquis and Savaysa.
If the Apple Watch user meets those requirements, they are emailed an "informed consent" document and some information related to the HIPPA privacy laws. Data from the study is expected to be used by Apple toward the development of a new product. In the consent papers, Apple says that some data could go toward determining whether sensors can identify irregular heart rhythms. Whatever new product the data is being mined for, it could come sooner than you might think. Apple has signed up with the FDA to be part of a program that allows the FDA to expedite the approval of certain devices through the regulatory agency.
Apple has also been working on a non-invasive method to measure blood sugar readings using sensors on the Apple Watch. Last December, this was said to be years away. Diabetics measure their blood sugar levels to see how much insulin they need to take. Currently, this is done by drawing a drop of blood from a finger, using a spring-loaded pen shaped device. The blood sample is placed on a test strip, which is inserted into a glucometer. The latter then displays the blood glucose reading on a screen. Apple could possibly save diabetics a lot of money if it can develop a way to get an accurate reading without requiring them to use expensive disposable test strips.
Source: Phonearena